Cygnus F030 BSA 残留检测试剂盒 BSA ELISA Kit (F030)
Cygnus试剂盒Cygnus technologies热销产品
Cygnus热销产品:cygnus f975、cygnus f410检测试剂盒、cygnus f550
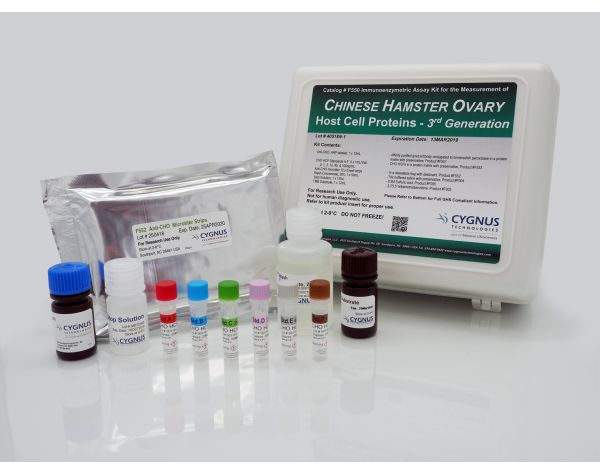
F015 CHO宿主蛋白残留检测试剂盒
F030 BSA残留检测试剂盒
F400 Protein A 试剂盒
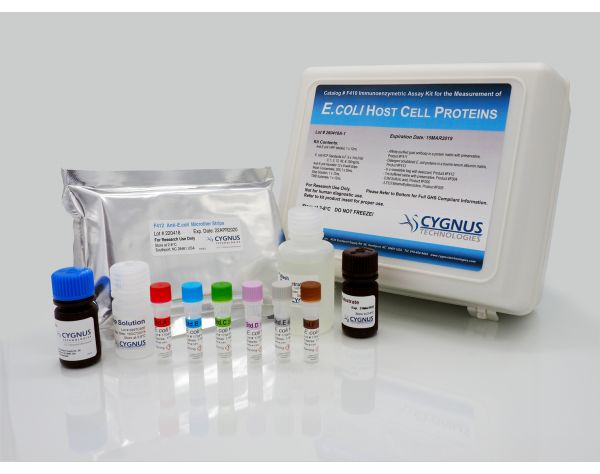
F410 大肠杆菌宿主蛋白残留检测试剂盒
CM015 CHO宿主蛋白残留检测试剂盒
F140 酵母菌宿主蛋白残留检测试剂盒
F150 HEK293宿主蛋白残留检测试剂盒
F050 蛋白A检测试剂盒
F500 Vero cell HCP ELISA试剂盒
品牌商名称:cygnus
公司官网:
Cygnus F030 BSA 残留检测试剂盒 BSA ELISA Kit (F030)
Product Information
Product Type: ELISA Kit
Storage: All kit reagents 2°C to 8°C
Assay format: 96 Well Plate
Time to result: ~1 hr. 50 min
LOD: ~125 pg/mL
LLOQ: ~250 pg/mL
Recommended Diluent: F031A
Description
This kit is intended for quantitating bovine serum albumin (BSA). The use of serum-free defined media greatly reduces the number of potential impurities, but it may still be necessary to determine the levels of trace impurities, such as proteins and growth factors used in these media. Most commercial formulations of serum-free media contain significant amounts of albumin and transferrin, either of bovine or human origin, and insulin from various species.
This highly sensitive assay for BSA has a detection limit of <250 pg/ml. It can be used to optimize purification processes and in routine quality control of in-process streams, as well as in testing the final product. Please use Sample Diluent, Item No. F031A, with these kits; available separately.
描述
该试剂盒用于定量牛血清白蛋白 (BSA)。 使用无血清成分的培养基大大减少了潜在杂质的数量,但仍可能需要确定微量杂质的水平,例如这些培养基中使用的蛋白质和生长因子。 大多数商业化的无血清培养基配方含有大量的白蛋白和转铁蛋白,无论是来自牛的还是来自人的,以及来自不同物种的胰岛素。
这种高灵敏度的 BSA 检测方法的检测限 <250 pg/ml。 它可用于优化纯化过程和过程中流的常规质量控制,以及测试最终产品。 请在这些套件中使用样品稀释剂,货号 F031A; 可单独购买。
Kit Components
Anti-BSA:HRP Conjugate
BSA Standards Set, A-E
TBS Wash Concentrate, 20X
Stop Solution
TMB Substrate for ELISA
Anti-BSA Coated Microtiter Strips
Cygnus其他相关试剂盒:
Cygnus I028 Sample Diluent Buffer
Cygnus F220 NS/0 HCP ELISA 试剂盒
Cygnus F050h Protein A-h ELISA 试剂盒
Cygnus F015 CHO HCP ELISA Kit 试剂盒 1 Kit
Cygnus F550 CHO HCP ELISA Kit, 3G 试剂盒 1 Kit
Cygnus CM015 CHO-CM HCP ELISA Kit 试剂盒 1 Kit
Cygnus F410 E.coli HCP ELISA Kit 试剂盒 1 Kit
Cygnus F500 Vero Cell HCP ELISA Kit 试剂盒 1 Kit
Cygnus F510 BHK HCP ELISA Kit 试剂盒 1 Kit
Cygnus F135 S.cerevisiae HCP ELISA Kit 1 Kit
Cygnus F020 SF9 HCP ELISA Kit 试剂盒 1 Kit
Cygnus F650 HEK 293 HCP ELISA Kit 试剂盒 1 Kit
Cygnus F490 L.lactis HCP ELISA Kit 试剂盒 1 Kit
Cygnus F300 MRC5 HCP ELISA Kit 试剂盒 1 Kit
Cygnus F650 HEK 293 HCP ELISA Kit 试剂盒 1 Kit
Cygnus F230 A549 HCP ELISA Kit 试剂盒 1 Kit
Cygnus F220 NS/0 HCP ELISA Kit 试剂盒 1 Kit
Cygnus F450 P.fluorescens HCP ELISA Kit 1 Kit
Cygnus F530 PER.C6 HCP ELISA Kit 试剂盒 1 Kit
Cygnus F140 Pichia pastoris HCP ELISA Kit 1 Kit
Cygnus F240 Total Goat Milk Proteins ELISA Kit 1 Kit
Cygnus F320 S.aureus HCP ELISA Kit 1 Kit
Cygnus F180 SP2/0 HCP ELISA Kit 试剂盒 1 Kit
Cygnus F500 Vero Cell HCP ELISA Kit 试剂盒 1 Kit
Cygnus F120 Bovine Transferrin ELISA Kit 试剂盒 1 Kit
Cygnus F030 BSA ELISA Kit 试剂盒 1 Kit
Cygnus F280 Insulin ELISA Kit, Ultra-Sensitive 1 Kit
Cygnus F045 Mouse IgG1 ELISA Kit 试剂盒 1 Kit
Cygnus F210 Goat IgG ELISA Kit 试剂盒 1 Kit
Cygnus F165 Human IgA ELISA Kit 试剂盒 1 Kit
Cygnus F070 Bovine IgG ELISA Kit 试剂盒 1 Kit
Cygnus F290 Bovine Plasma Proteins ELISA Kit 试剂盒 1 Kit
Cygnus F160 Human IgG, Total, ELISA Kit 试剂盒 1 Kit
Cygnus F170 Human IgM ELISA Kit 试剂盒 1 Kit
Cygnus F055 Human Serum Albumin ELISA Kit 试剂盒 1 Kit
Cygnus F035N Human Transferrin ELISA Kit 试剂盒 1 Kit
Cygnus F050 Protein A ELISA Kit 试剂盒 1 Kit
Cygnus F050H Protein A-h ELISA Kit (human IgG) 1 Kit
Cygnus F040 Insulin ELISA Kit 试剂盒 1 Kit
Cygnus F046 Mouse IgG2a ELISA Kit 试剂盒 1 Kit
Cygnus F047 Mouse IgG2b ELISA Kit 试剂盒 1 Kit
Cygnus F090 Mouse IgM ELISA Kit 试剂盒 1 Kit
Cygnus F049 Mouse Total IgG ELISA Kit 试剂盒 1 Kit
Cygnus F400 Protein A ELISA Kit 试剂盒 1 Kit