| Poduct Number | Preps | Price |
|---|---|---|
| R6874-00 | 5 | ¥130 |
| R6874-01 | 50 | ¥990 |
| R6874-02 | 200 | ¥3480 |
参数
组分
流程

| Poduct Number | Preps | Price |
|---|---|---|
| R6874-00 | 5 | ¥130 |
| R6874-01 | 50 | ¥990 |
| R6874-02 | 200 | ¥3480 |
| Poduct Number | Preps | Price |
|---|---|---|
| D3892-00 | 5 | ¥108 |
| D3892-01 | 50 | ¥715 |
| D3892-02 | 200 | ¥2640 |
Catalog ID
Boost viral vector production in HEK293 cells up to 10-fold.
Details
Gene therapy and viral vector-based vaccine manufacturers rely on basal media as the standard to grow their adeno-associated (AAV) viral vectors. The first-of-its-kind BalanCD HEK293 Viral Feed from FUJIFILM Irvine Scientific delivers a 3-fold to 10-fold increase in viral vector production over basal media alone to provide consistent performance, scalability, and speed time-to-market.
BalanCD HEK293 Viral Feed can be used with HEK293 suspension cultures from small- to large-scale for AAV production of multiple serotypes, as well as a range of agnostic basal growth media and transfection methods.
BalanCD HEK293 Viral Feed promotes vigorous VG/capsid yields and multiple-fold increases in titers to help manufacturers get more working volume for their applications.
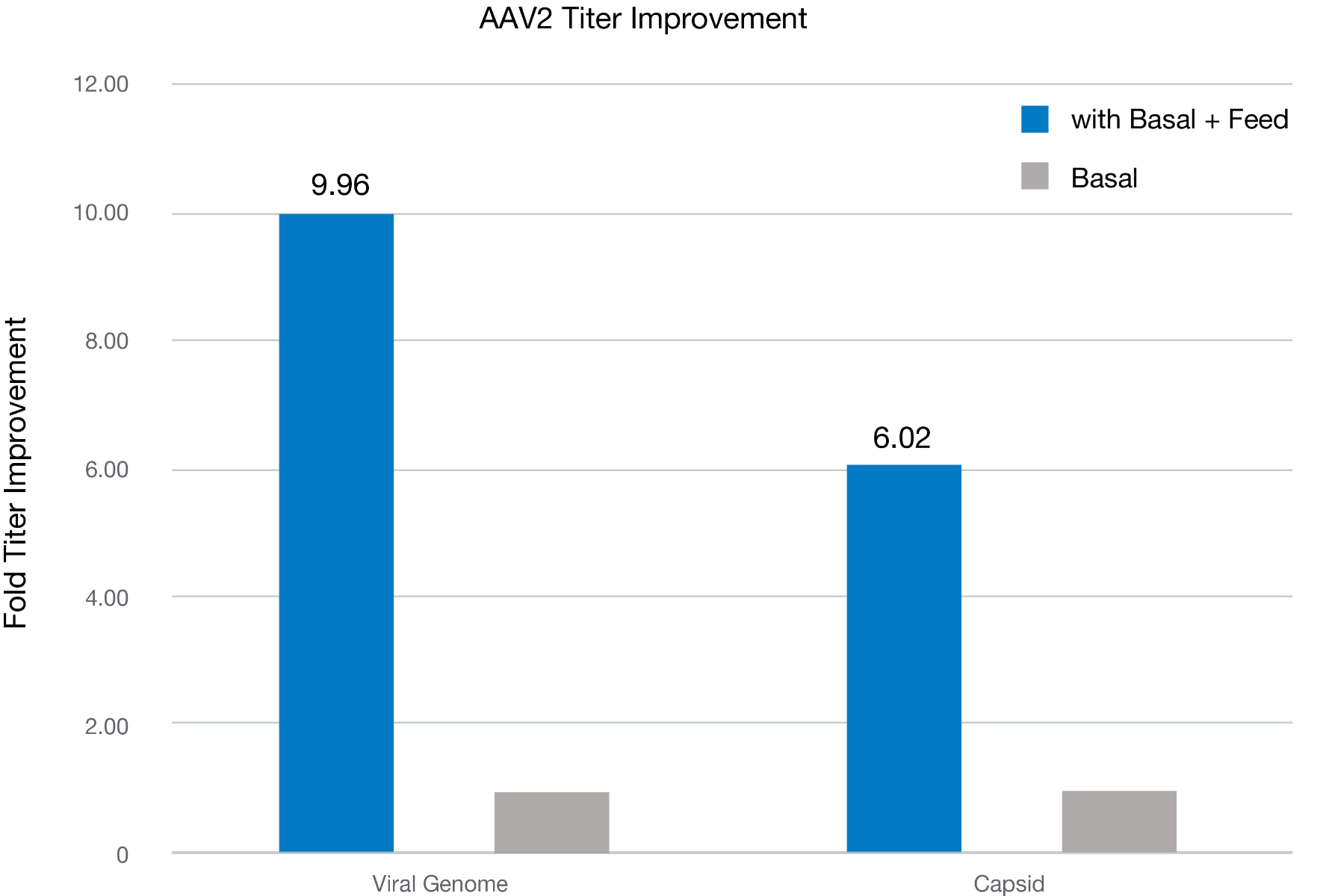
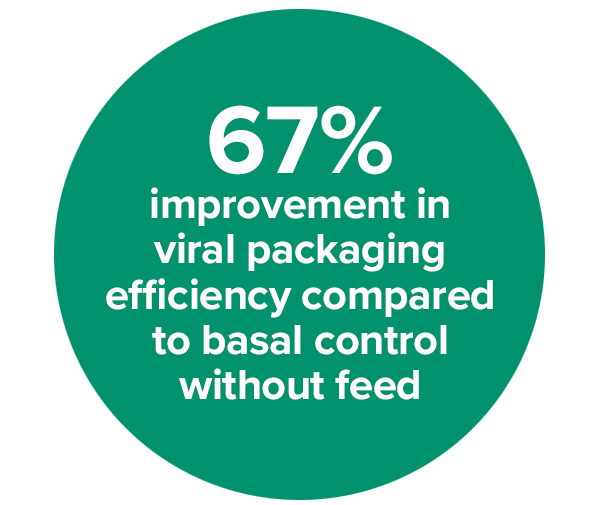
Figure 1. AAV2 viral genome titer and capsid titer were measured using cell extracts subjected to three cycles of freeze-thaw, followed by clarification by centrifugation and treatment with DNAse I and lysis buffer prior to qPCR (using AAVpro titration kit, Takara Bio) and ELISA (AAV2 titration ELISA, Progen). Blue and orange bars indicate the viral genome (VG) and capsid titer fold over the basal culture without a feed. The data indicates that the feed media enhancing the AAV2 production approximately 10-fold in VG and 6-fold for the capsid titer. As an overall result, viral packaging was determined to improve about 67%, from 13% in Basal to 21.81% in Basal+feed (data not shown).
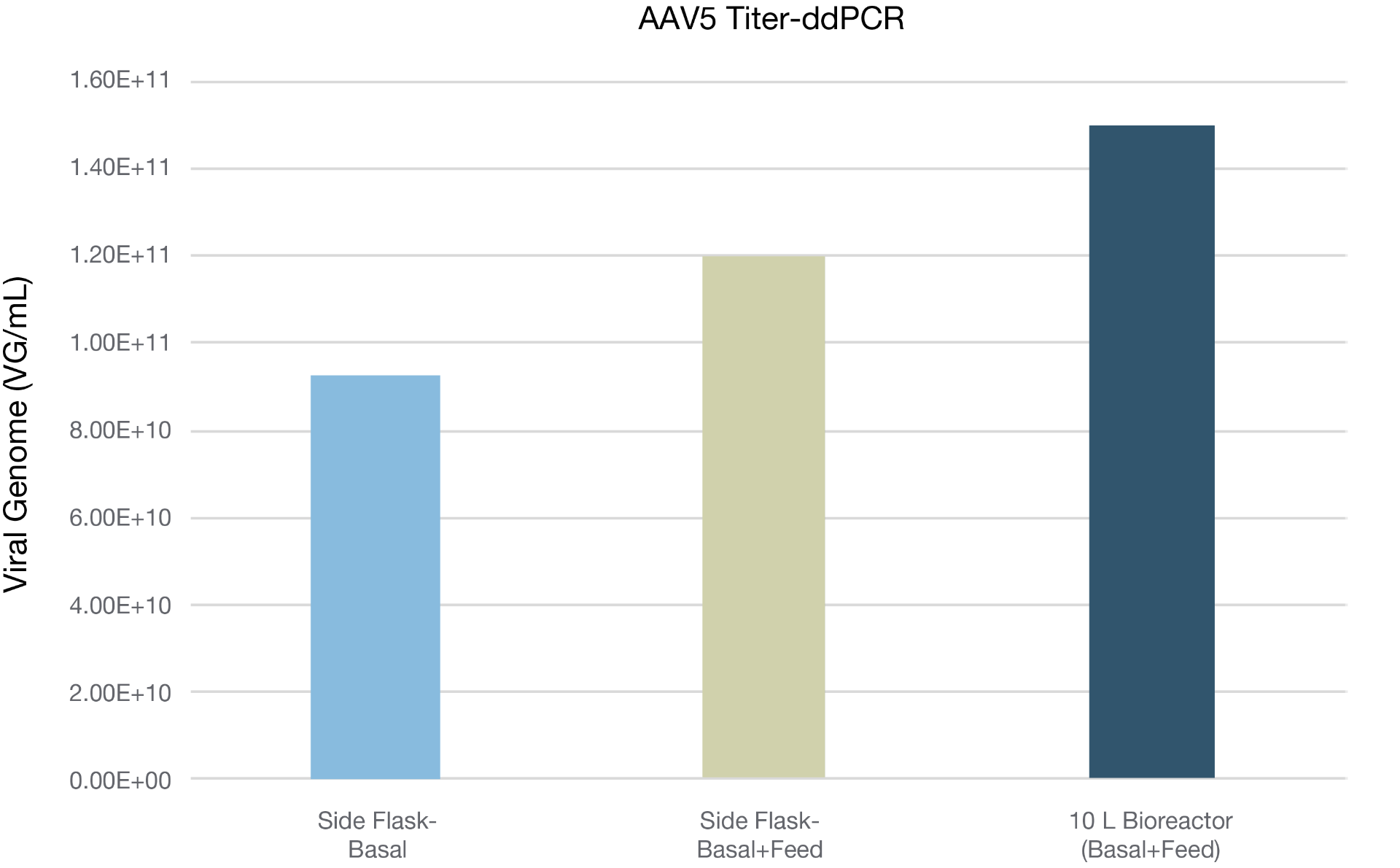
Figure 2. Verification of the BalanCD HEK293 Viral Feed performance on improving the production of AAV5 in 10L bioreactor scale. HEK293 suspension cells (cell line 2) were seeded at density of 0.8X10^6 cell/mL in 8.3 L culture volume in 10L bioreactor (XDR10 bioreactor). BalanCD HEK293 Viral Feed was added at 12% v/v at 24 hr post transfection. Titer was measured using ddPCR.
BalanCD HEK293 Viral Feed is manufactured in a GMP facility using qualified raw materials sourced from a solid supply chain to ensure continuity of supply and lot-to-lot reliability for HEK293-specific applications. Our stringent oversight provides confirmation of formula, analysis, and assurance that BalanCD HEK293 Viral Feed meets the highest global and regional standards while fulfilling regulatory demands with each manufacturing lot file.
for other packaging sizes and powder format availability.
上海金畔生物代理FUJIFILM Irvine Scientific富士胶片欧文科技细胞培养基,部分现货,量多优惠,欢迎来电咨询18301939375!
FUJIFILM Irvine Scientific富士胶片欧文科技特色产品
BalanCD™ CHO Platform/培养基平台
BalanCD™ HEK293 培养基平台
PRIME-XV免疫细胞培养基
PRIME-XV干细胞培养基
冻存液和其它
免责声明
1. 本公司密切关注本网站发布的内容,但不保证发布内容的准确性、完整性、可靠性和最新性等。
2. 本公司不保证使用本网站期间不会出现故障或计算机病毒污染的风险。
3. 无论何种原因,使用本网站时给用户或第三方造成的任何不利或损害,本公司概不负责。此外,对于用户与其他用户或第三方之间因本网站发生的任何交易、通讯或纠纷,本公司概不负责。
4. 本网站可提供的所有产品和服务均不得用于人体或动物的临床诊断或治疗,仅可用于科研等非医疗目的。如任何用户将本网站提供的产品和服务用于临床诊断或治疗,以及其他特定的用途或行为,本公司概不保证其安全性和有效性,并且不负任何相关的法律责任。
Biolegend官网:https://www.biolegend.com/
Cosmobio官网:http://www.cosmobio.co.jp/
Harlan官网:https://www.envigo.com/
Jackson官网:https://www.jacksonimmuno.com/
Lumiprobe官网:https://www.lumiprobe.com/